A new experimental therapy that is based on a series of electrical implants that are placed in the spinal cord and a device that is responsible for modulating an electrical signal has managed to stimulate the ability to move in 16 children with cerebral palsy, as shown by the results from a pilot study that has been published in Nature Communications.
The researchers who have carried out the trial have explained that all the children who have been treated with this technology have experienced improvements between four and eight weeks after starting the therapy, with which it has been possible to “improve the sensory and motor function” of patients between the ages of two and 16 with different degrees of paralysis, something that is the first time that it has been achieved in children, they say. The new therapy has been developed by Dr. Susan Hastings and Dr. Reggie Edgerton.
This is the first pilot study carried out with this type of technology in children and has been led by the biomedical company ‘SpineX’. The same tool has been used with which three paraplegic adults due to an accident had already been able to walk, run and carry out other activities using electrical implants. This case is different, since the children in whom movement has been stimulated had not previously had the ability to move, as was the case with the adults in the previous study.
The new technology has managed to “improve sensory and motor function” in children aged two to 16 with cerebral palsy between four and eight weeks after starting therapy
“My son is now able to sit and stand upright more stable than before, he has more control of his head and has even improved his ability to observe what is happening around him,” said the mother of one of the treated children who have participated in this pilot study.
How experimental therapy works in children with cerebral palsy
Cerebral palsy is a disease of which around 10,000 new cases are diagnosed each year and which occurs due to damage to the brain during prenatal development. Cerebral palsy symptoms are characterized by movement disorders that affect the patient’s ability to move and maintain balance and posture. There are no treatments to prevent or cure the disease.
Dr. Josep M. Tormos, director of research at the Guttmann Neurorehabilitation Institute, explained in an interview with El Periódico de Catalunya how this technology works. According to this independent expert, one of the functions of the nervous system is to control a series of reflexes so that the body functions properly. In the case of movement, our brain not only indicates when and how we move a leg, but also gives orders to inhibit movements. But, when an injury occurs, this process is altered, and therefore people who have suffered spinal cord injuries lose control of their body, and not only can they lose part of their mobility, but they can also develop involuntary movements.
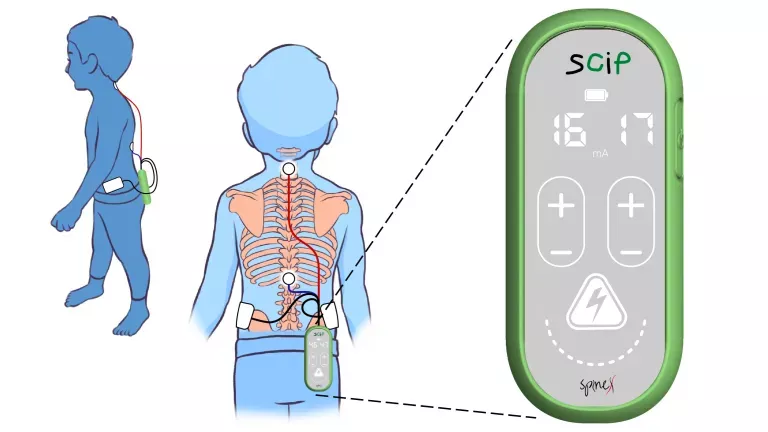
Electrode stimulation techniques intervene in these processes that have deteriorated to try to “reconfigure the rules of the game”, indicates Dr Tormos. In the case of injured adults, the objective of the implants is to restore the neuronal connections that have been lost. In children with cerebral palsy, however, the challenge is greater, since it is necessary to create signals that had not existed until now. “The study presented this Wednesday marks yet another milestone in the development of this type of technology,” says the specialist.
This new electrode therapy for children with cerebral palsy is in the study and development phase. The American Food and Drug Administration (FDA) has included the SpineX device in its technological innovation program to promote research on this product and speed up the process for its approval. The intention of its creators is to start a clinical trial in 2023 and, if the results confirm its benefits, they will request official authorization for the treatment of cerebral palsy in children, although this process could take years.
.




